Hotspots for allosteric control
Molecular Systems Biology 2011
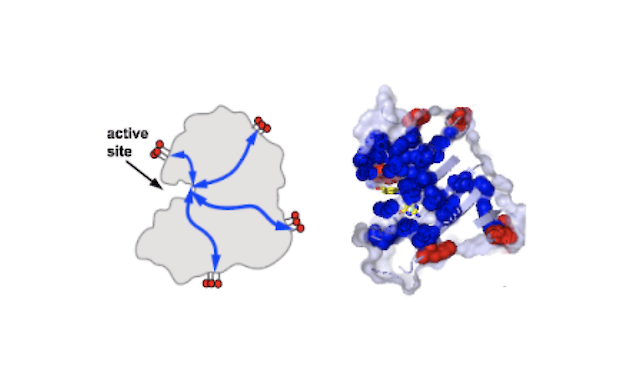
Sectors are coevolving groups of amino acids within the tertiary structure of proteins that typically form physically-connected networks that link a few distantly positioned surface sites to the main functional site. In some cases, this architecture seems to explain known allosteric mechanisms, but interestingly, it is also found in proteins with no known allostery. In these cases, the prediction is that sector-connected surface sites should represent cryptic allosteric sites – capable of accepting regulatory input by simply linking an input signaling module. In this work, we carry out a systematic “domain-insertion scan” as a deep test of this hypothesis. Specifically, we insert a plant light-sensing domain (as-LOV2) into every surface-exposed site in E. coli dihyrdrofolate reductase (72 total), and examined each site-specific fusion for light-dependent DHFR activity in vivo. The bottom line is that 14 out of 72 sites show statistically significant light-dependence, and every one of these 14 sites occurs at the surface edge of the protein sector. Thus, we propose that sector connected surface sites are “hot spots” for the emergence of new regulation in proteins, with sectors themselves serving as the communication mechanism. We synthesize these results with prior work to produce a model for the de novo evolution of communication between proteins.